The draft manuscript describing our COVID-19 surveillance clinical study was posted ahead of print on medRxiv nearly a year ago. We are delighted to let you know that the peer-reviewed article is now published in mSphere. Congratulations to the authors!
Longitudinal COVID-19 Surveillance and Characterization in the Workplace with Public Health and Diagnostic Endpoints
On March 19, 2020, businesses and institutions across the state of California closed and locked their doors in compliance with the stay-at-home orders enacted to slow the spread of COVID-19. Meanwhile, at Oak Crest, the employees donned masks and waited patiently in their cars to get tested for the SARS-CoV-2 virus. Over 1 year and 4,000 swabs later, we are proud to announce that our ongoing dedication to regular workplace COVID-19 testing has been immensely successful in keeping everyone at Oak Crest safe and the institute operational, and in generating unique scientific data published in this and future articles.
This accomplishment is a culmination of thousands of hours of planning, benchwork, and analysis which has kept Oak Crest productive throughout the pandemic. Due to our testing, we have successfully prevented workplace transmission of COVID-19 by identifying and isolating positive cases before allowing the virus to spread. Because our participants are continuously tested and have volunteered for blood draws, we are assembling a unique dataset on qPCR test positivity correlated with other biomarkers such as antibodies.
Some of the central takeaways:
-
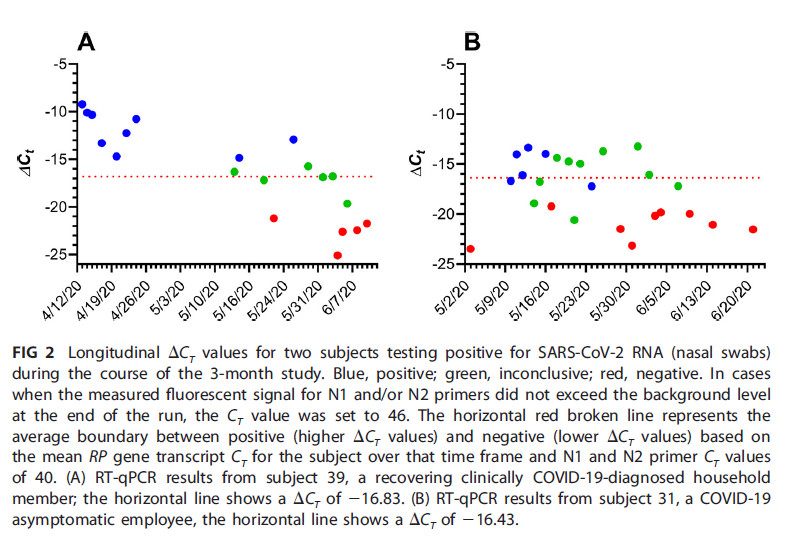
Everyone reacts differently to COVID-19 infection, both in terms of genome copy number and duration of testing positive by qPCR. One participant tested positive for 71 days.
-
Participants differ in their antibody response as well, with different levels of immunoglobulin M (IgM, immediate response), immunoglobulin A (IgA, mucosal immunity) and immunoglobulin G (IgG, blood).
-
Antibody levels drop rapidly after clearing the infection, with half-life of 1.3 days (for IgA).