Oak Crest Scientists Develop Novel Subdermal Implant for the Prevention and Treatment of HIV/AIDS
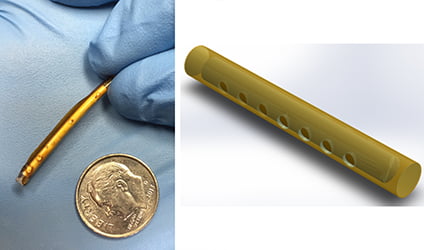
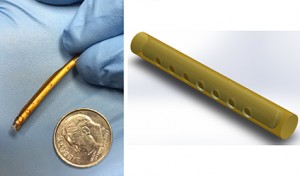
Findings published this week in Antimicrobial Agents and Chemotherapy report that a novel, subdermal implant delivering potent antiretroviral (ARV) drugs shows extreme promise in stopping the spread of HIV. Scientists from the Oak Crest Institute of Science, in Pasadena, CA, report that they have developed a matchstick size implant, similar to a contraceptive implant, that successfully delivers a controlled, sustained release of ARV drugs for up to 40 days in dogs with no adverse side effects. “To our knowledge this is the first implant to be used for this purpose,” says Dr. Marc Baum, Senior Faculty and founder of Oak Crest.
Daily administration of oral or topical ARV drugs to HIV negative individuals in vulnerable populations is a promising strategy for HIV prevention. However, adherence to the dosing regimen has emerged as a critical factor in determining effective outcomes in clinical trials.
“This novel device will revolutionize how we treat or prevent HIV/AIDS as it delivers powerful HIV-stopping drugs and eliminates one of the key obstacles in HIV/AIDS prevention – adherence to proper dosing regimens,” he adds.
Medical practitioners and scientists acknowledge that one of the main drawbacks in current medical treatments is the problem of adherence. “It’s unfortunate, but patients do not always follow the dosing instructions as prescribed,” says Dr. Baum, “in clinical trials erratic administration of drugs has led to highly variable efficacy outcomes. That’s what peaked our interest in the possible use of a subdermal implant for the prevention of HIV,” suggests Dr. Baum.
The use of subdermal implants for contraception began in the United States in 1993. The small flexible tube, measuring about 40mm in length, is inserted under the skin by a health care professional (typically the upper arm). After it is inserted it prevents pregnancy by releasing hormones that prevent ovaries from releasing eggs and by thickening cervical mucous.
“Our subdermal implant is used in the same manner as a contraceptive implant. It is easily inserted and removed and provides sustained release of the potent prodrug tenofovir alafenamide, which is roughly ten times more potent against HIV than tenofovir disoproxil fumarate, another tenofovir prodrug that has been shown to prevent sexually transmitted HIV when used as a pre-exposure prophylaxis,” adds Dr. Baum. “We are very pleased with the results of our preliminary studies and are working diligently to develop a subdermal implant for HIV prevention that will remain effective for a full 12 months.”